Many students remain on campus over the summer to do research with Juniata faculty. We have had as many as 50 students doing paid summer research in the various departments. Many students also do research during the academic year, either for extracurricular experience or for credit. In addition, some students spend summers at other sites doing research. Recent students have been at federal labs at Oak Ridge and Argonne, academic labs at Harvard, Utah, Virginia, and Berkeley, and industrial labs such as Restek.
Most Juniata chemistry graduates do research at some point during their undergraduate careers, and many present their work at outside conferences. Juniata always has one of the largest contingents at the annual National Conference on Undergraduate Research, and each year a group of students and faculty attend the spring American Chemical Society meeting and present posters there.
Recent Student Research
- Angela Wilkins '20, Isabella Weber '20, and Nicodemus Usry '19: "Poppy Seed Consumption and Associated Morphine Levels for Urine and Saliva Samples."
- Tyler Weigel '19: "Preparation of Chiral Aziridines Through a Copper-Catalyzed Asymmetric Reduction of 2H-Azirines."
- Katie Goerl '19: "Synthesis of 2-Pyridinecarbaldehyde N-Oxide."
- Michael Fox '19: "Synthesis of Polythiopene Nanoparticles for Astrochemistry Research."
- Jessica Zavadak '18: "Differential Gene Expression in Alzheimer's Disease Mouse Model."
- James McGettigan '18: "The Synthesis of Selectively Deuterated Tetra(benzyloxy)benzenes for Mass Spectrometry Studies."
Current Faculty Research
Dr. Ames
Research within the Ames lab focuses on utilizing methods and insight from a number of chemical sub-disciplines. Most projects require knowledge of the organic, inorganic, analytical, physical, and biochemical sub-disciplines. Students working within the group have been involved in organic synthesis, inorganic transition metal complexation, analytical electrochemical measurements, thin film materials analysis, quantum mechanical calculations of reaction mechanisms, and molecular dynamics simulations of biomolecules to name a few.
The major thrust of our research is the investigation of porphyrin molecules as catalysts – either for water oxidation, or for carbon dioxide reduction – both important topics relating to sustainable clean energy research.
Dr. Ames also has interest in the spectroscopy and reactivity of bioinorganic molecules and inorganic model complexes, as well as the characterization of radical enzyme intermediates.
In working with Dr. Ames, and other group members, it is the goal that student researchers will become full owners of their projects. Moving from the primary role of student to the role of colleague, an equal partner in the research being done.
Dr. Baran
Our research is focused on the synthesis and characterization
of coordination compounds with applications as:
1. catalysts in organic synthesis,
2. new magnetic materials, or
3. complexes with interesting biological properties.
We also study metal complexes in plant products, such as grape wine, and interactions between metallic ions and organic compounds found in plants.
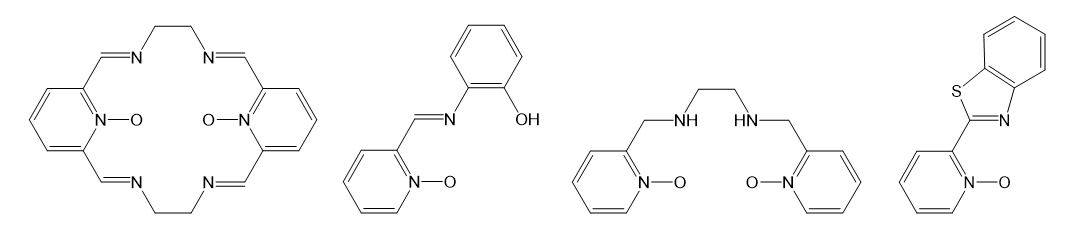
A common feature for most of our projects is the implementation of aromatic amine N-oxides as organic ligands and building blocks for transition metal coordination compounds. Besides the use of some classical N-oxides, for example pyridine N-oxide or 1,10-phenanthroline N-oxide, we also synthesize new derivatives of N-oxides to fine-tune their chemical and physical properties. As far as for metals, mostly complexes with first row transition metals are explored.
Composition and structure of the organic ligands and inorganic coordination compounds are studied by spectroscopic methods and X-ray crystallography available in our department. We maintain collaborations on study of magnetic properties and biological activity of prepared complexes with other US institutions as well as international partners in Slovakia, Czech Republic, and Germany. Undergraduate students from our group present their research at various venues including the national ACS meetings and international conferences abroad. Our accomplishments have been published in peer-reviewed journal such as Inorganic Chemistry, Inorganica Chimica Acta, Acta Crystallographica and more publications are coming soon.
A series of new polydentate Schiff base ligands containing pyridine N-oxide fragment(s), such as those shown below, were prepared in order to study Transition Metal Complexes for Catalysis in Organic Oxidations.
Ability of the N-oxide group to bridge between two metallic centers predetermines N-oxides to act as bridging ligands in the formation of multinuclear complexes. At the appropriate composition, some of these exhibit properties reminiscent of Single Molecule Magnets. In the case of ferromagnetic interactions between metallic centers, the overall effective magnetic moment adopts much bigger values than those in mononuclear complexes, predetermining such complexes to be studied for use as non-gadolinium based Magnetic Resonance Imaging Contrast Agents.
Purine N-oxides, such as guanine 3-N-oxide, we use as organic ligands in coordination compounds with potential Anticancer Activity.
Prevention of skin allergies caused by urushiol, an allergen found in Poison Ivy and several other plants, is studied using model compounds as well as urushiol itself. Interaction of urushiol with metallic salts shows promises to an effective prevention of the penetration of the urushiol through the skin to the blood stream.
Some natural systems such as wine represent an extremely complex equilibrium of hundreds of organic compounds (sugars, alcohols, carboxylic acids, phenolics, esters, aldehydes, amines, …) interacting with dozens of metallic cations creating possibly thousands of coordination compounds. Our group is trying to open a door into this fascinating yet completely unmapped territory of Wine Chemistry.
Dr. Dries
Our lab is interested in neurological diseases, particularly Alzheimer’s disease. We take a variety of approaches, ranging from enzymatic assays to microscopy. I believe that students learn best from one another. While I have weekly meetings with all students, I rely on the students to teach the various techniques to one another, further cementing their understanding in the lab. Undergraduate researchers have presented their work in a variety of ways, including at national meetings and as theses. For more information, please visit our lab webpage to learn who we are and what we do!
Dr. Williams
Inorganic metals are at work all around us, from the enzymes that function in biology
to the magnets that power our cell phones to the catalysts that are used in chemical
industry. The goal of research in our lab is to better understand how a metal ion’s
environment affects its properties in order to better understand the roles that metals
play in a variety of applications. To investigate this topic, students in the lab
perform organic and inorganic synthesis and make use of a wide variety of analytical
techniques. Undergraduate students working in the lab learn the value of collaboration
and challenge themselves to solve difficult problems. Students also have the opportunity
to present their research at a variety of on- and off-campus venues.
Dr. Yohn
Chemistry doesn’t just happen in the laboratory – it happens all around us. Environmental geochemistry involves the integration of chemistry with biology, geology and physics to collect, interpret, and apply information about aquatic and terrestrial natural systems. Much of our work focuses on the local 28 mile long reservoir, Raystown Lake, including a long term monitoring program evaluating the health and trophic status of the lake, as well as investigating the relationship between nutrients and the spread of invasive aquatic plants. Students in this group combine field and lab work to answer specific applied questions about natural systems, as well as developing analytical techniques to quantify relevant chemicals such as chlorophyll or organic contaminants.